Curriculum
Stoichiometric Relationship
INTRODUCTION TO THE PARTICULAR NATURE OF MATTER AND CHEMICAL CHANGE
0/2ASSIGNMENTS
0/2MOLE CONCEPT
0/2ASSIGNMENTS
0/2REACTING MASSES AND VOLUMES
0/2ASSIGNMENTS
0/2States of Matter and changes in phases
States of matter
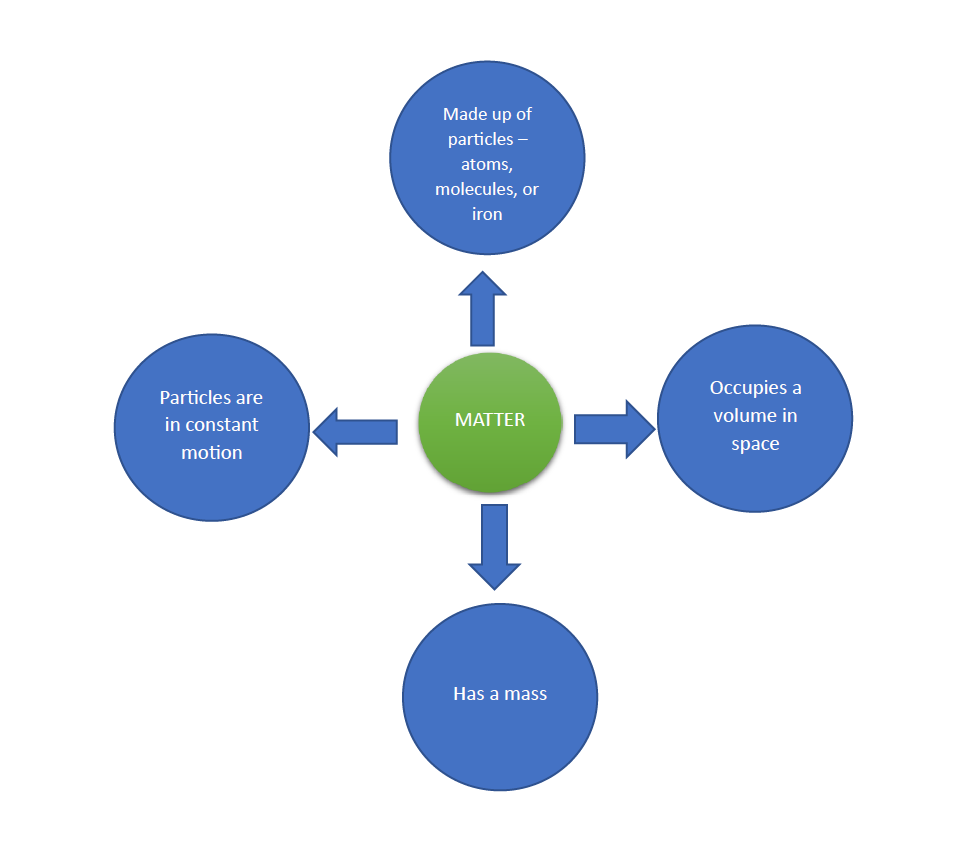
Matter is everywhere. We are made up of matter, we consume it, it surrounds us, and we can see and touch many forms of matter. Air is a form of matter which we know is there, though we cannot see it. Our planet and the entire universe are made up of matter and chemistry seeks to expand our understanding of matter and its properties.
![]()
![]()
The properties of the three states of matter are summarized below :
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The way the particles of matter move depends on the temperature. As the temperature increases the average kinetic energy of the particles increases – the particles in a solid vibrate more. The particles in liquids and gases also vibrate, rotate, and translate more.
Temperature
There are a number of different temperature scales. The most commonly used are the Fahrenheit, Celsius, and Kelvin scales. All three are named in honour of the scientist who developed them.
The SI unit for temperature is the kelvin (K). The Kelvin scale is used in energetics calculations (see topic 5).
Absolute zero is zero on the Kelvin scale, 0 K (on the Celsius scale this is -273 °C). It is the temperature at which all movement of particles stops. At temperatures greater than absolute zero, all particles vibrate, even in solid matter.
You can convert temperatures from the Celsius scale to the Kelvin scale using the algorithm :
temperature (K) = temperature (°C) + 273.15
Changing States of Matter
You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Changing states of matter occur when matter loses or absorbs energy. When a substance absorbs energy; the atoms and molecules move more rapidly and this increased kinetic energy pushes particles far enough that they change form. This energy is usually heat or thermal energy. In this article, let us understand the science behind the changing states of matter.
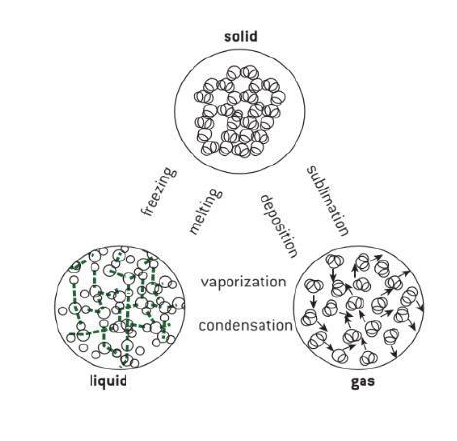
Why do Phase Changes Occur?
When temperature or pressure change of a system occurs, phase changes occur. When the temperature or pressure increases, the interaction between the molecules increases. Similarly, when the temperature decreases, it is easier for molecules and atoms to settle into a more rigid structure.
Changes Between Liquids and Solids
How would you make ice cubes in a tray? First, you would fill the tray with water from a tap. Then you would place the tray in the freezer compartment of a refrigerator. The freezer is very cold. What happens next?
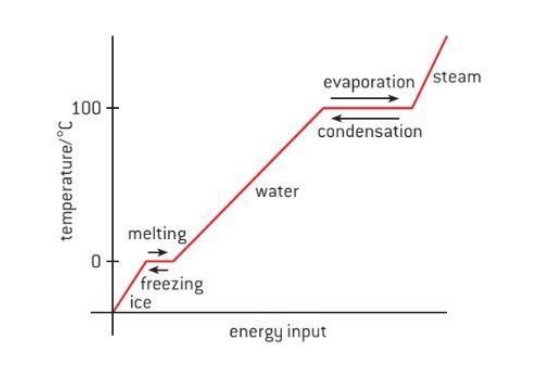
Freezing
Heat transfer occurs between the warmer tray and the colder air in the freezer. The warm water loses heat to the cold air in the freezer. This heat transfer occurs until no energy is available for the particles to slide past each other. This forces them to remain in fixed positions, locked in place by the force of attraction between them. This way liquid water is changed into solid ice. The process of liquid water changing to solid ice is termed as freezing. The temperature at which it occurs is known as the freezing point.
Melting
If you took out the ice cubes from the freezer and placed them in a warm room, the ice would absorb energy from the warmer air around them. This absorbed energy would facilitate them to overcome the force of attraction holding them together, enabling them to slip out of the fixed position that they held as ice. The process in which a solid change to a liquid is called melting. The melting point is the temperature at which solids change to a liquid.
Changes Between Liquids and Gases
If you fill a pot with cold tap water and heat it on a hot stovetop, the water heats up. Heat energy travels from the stovetop to the pot, and the water absorbs the energy from the pot. What happens to the water next?
Vaporization
If the water is hot enough, it starts to boil. Bubbles of water vapor are formed in the boiling water. This happens as particles of liquid water gain enough energy to completely overcome the force of attraction between them and change to the gaseous state. The bubbles rise through the water and escape from the pot as steam. The process in which a liquid boils and changes to a gas is called vaporization. The temperature at which a liquid boils, is its boiling point.
Condensation
When you take a hot shower in a closed bathroom, the mirror is likely to fog up. You may wonder why does this happen? Some hot water from the shower evaporates and when it comes in contact with cooler surfaces such as the mirror, it cools and loses energy. The cooler water particles no longer have the energy to overcome the forces of attraction between them. They come together and form droplets of liquid water. This process in which a gas changes to liquid is known as condensation.
Changes Between Solids and Gases
Solids that change to gas pass through the liquid state first. However, sometimes solids change directly to gases and skip the liquid state. The reverse can also occur. Sometimes gases change directly to solids.
Sublimation
The process in which solids directly change to gases is known as sublimation. This occurs when solids absorb enough energy to completely overcome the forces of attraction between them. Dry ice is an example of solids that undergo sublimation.
Five Changes of State are:
• Melting
• Freezing
• Evaporation
• Condensation
• Sublimation
• Deposition
The process by which a substance changes from the solid phase to the liquid phase is known as melting.
The process by which a substance changes from the liquid phase to the solid phase is known as freezing.
The process by which a substance changes from the liquid phase to the gaseous phase is known as evaporation. The process by which a substance changes from the gaseous phase to the liquid phase is known as condensation. The transition of the solid phase to the gaseous phase without passing the intermediate liquid phase is known as sublimation.
Deposition
The opposite of sublimation is deposition. Which is the process of a gas changing directly into a solid, bypassing the liquid state. For example, when water vapor in the atmosphere turns directly into ice, like frost, that’s deposition.