Stoichiometric Relationship
- Description
- Curriculum
- Reviews
INTRODUCTION TO THE PARTICULAR NATURE OF MATTER AND CHEMICAL CHANGE
-
1Elements, Compounds and Mixtures
An element is a substance that consists of identical atoms or molecules with only one type of atomic core and a compound is a substance that consists of different elements in definite proportions while a mixture is a material made up of two or more different chemical substances which can be separated by physical methods.
-
2States of Matter and changes in phases
Matter can exist in three primary phases : solids, liquids and gases.
A phase change or phase transition is a change among thsee states of matter.
ASSIGNMENTS
MOLE CONCEPT
-
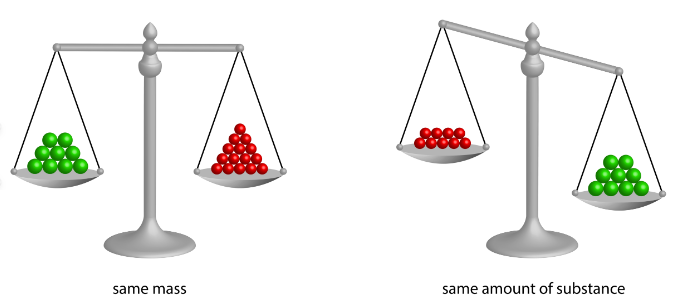
5What is Mole Concept ?
The mole concept is a convenient method of expressing the amount of a substance.
-
6Empirical Formulae and Molecular Formulae
Empirical formulae give the simplest ratio of different atoms in a compound where Molecular formulae give the actual number of each different atom in a molecule.
ASSIGNMENTS
REACTING MASSES AND VOLUMES
-
9Limiting Reagents, Theoretical and Experimental Yields, Volumetric Analysis
- Balancing a chemical reaction
- Atom Economy
- Limiting Reagent
- Theoretical and Experimental Yield
- Concentration
- Volumetric Analysis
-
10Avogadro's Law and Other Gas Laws
- Boyle's Law
- Charle's Law
- Gay-Lussac's Law
- Combined Gas Law
- Ideal Gas Law
- Avogadro's Law
ASSIGNMENTS
Please, login to leave a review