Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
0/1TOPIC – 01 : Order of Boiling Point in Alkanes
The physical properties of the members of a homologous series change gradually as the length of the carbon chain increases. For example, the boiling points of members of the alkane series can be measured using a certain apparatus. Such an experiment shows that the boiling point rises with an increasing number of carbon atoms (or increasing molar mass). This can be seen by the state at room temperature within the series: butane is a gas at room temperature, while pentane is a liquid.
The homologous series of alkane is giving below along with their corresponding boiling points. 
This trend in boiling point results from increasingly strong intermolecular forces (London (dispersion) forces) as the carbon chain becomes longer. Trends in increasing density and viscosity with carbon chain length are well understood by the petrochemical industry. Crude oil is a mixture of hydrocarbons that vary in the length of their carbon chain. Fractional distillation is a physical separation process that uses differences in boiling points to separate the mixture into fractions of similar boiling point. A simple distillation apparatus can effectively separate volatile fractions from long-chain, non-volatile compounds in a school laboratory.
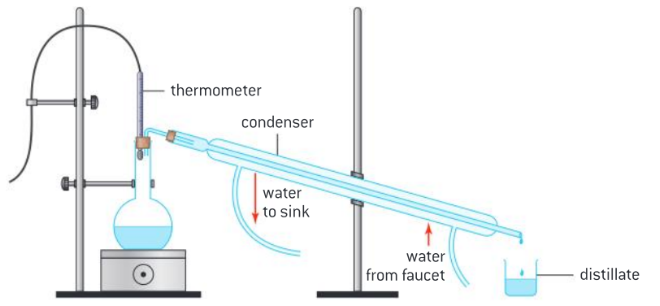
Figure : 1 Distillation apparatus incorporating a temperature probe