Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
0/1
Text lesson
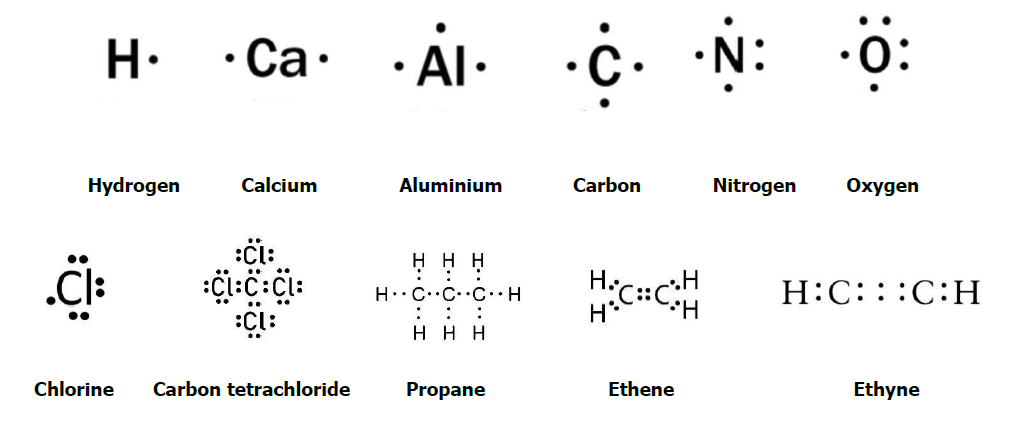
TOPIC – 04 : Lewis Dot Structural Formulae
Lewis formulas are structures that show the connectivity on bonding sequence of the atoms, indicating single, double or triple bonds. They should always show any formal changes and unshared electrons that might be present in the molecule. Mainly this formula shows only valance electrons in the form of dots.
Lewis structures are a useful way to Summarize Certain information about bonding and may be thought of as “electron bookkeeping”. In Lewis dot structures each dot represents an electron. A pair of dots between chemical symbols for atoms represents a bond. This formula or structure was named after Gibert N. Lewis who introduced this to us.
Example :