Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
0/1TOPIC – 04 : Classifying molecules : Primary, Secondary, Tertiary and Quaternary compounds
How To Recognize Primary, Secondary, Tertiary and sometimes Quaternary Hydrocarbons, Carbocations, Alkyl Halides, Alcohols, Amines, and Amides :
¨ Primary (1°), secondary (2°), tertiary (3°) and quaternary (4°) alkyl carbons are defined according to the number of carbons directly attached to that carbon.
¨ Similar nomenclature can be used for carbocations. Primary (1º), secondary (2°), and tertiary (3°) carbocations are defined according to the number of carbons directly attached to the carbon bearing the positive charge.
¨ Primary (1°), secondary (2°), and tertiary (3°) alcohols are defined according to the number of carbons directly attached to the carbon bearing the hydroxyl group.
¨ Primary (1°), secondary (2°), and tertiary (3°) alkyl halides are defined similarly to alcohols.
¨ Amines and amides are defined according to the number of carbons directly attached to the nitrogen atom.
1. Primary, Secondary, Tertiary, and Quaternary Alkyl Hydrocarbons
There are four possible bonding patterns for alkyl carbons in hydrocarbons.
¨ Primary carbons (1°), are carbons attached to one other carbon and three hydrogens. Also known as a methyl (CH3)
¨ Secondary carbons (2°) are attached to two other carbons and two hydrogens. Also known as methylene (CH2) carbons.
¨ Tertiary carbons (3°) are attached to three other carbons and one hydrogen. Also known as methine (R3CH) carbons.
¨ Finally, quaternary carbons (4°) are attached to four other carbons.
We can’t go higher than that. To have five substituents, 10 electrons around carbon are required, a clear violation of the octet rule. Writing 5 covalent bonds around one carbon will count as a mistake.
Alkyl carbons are classified as primary, secondary, tertiary or quaternary according to the number of directly attached to the carbon in question.
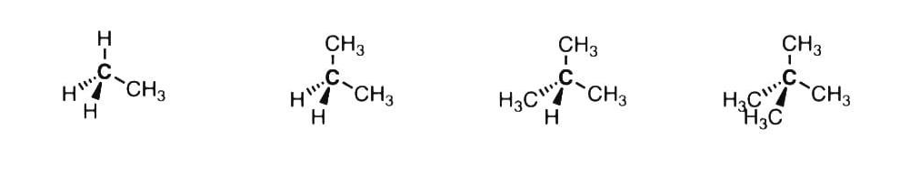
|
Primary (1°) carbon |
Secondary (2°) Tertiary (3°) Quaternary (4°) carbon carbon carbon |
|
“methyl”
|
“methylene” “methine” “quaternary” |
|
-C attached to one carbon |
-C attached to -C attached to -C attached to two carbons three carbons four carbons
|

It’s important to note that the terms primary, secondary, tertiary and quaternary only apply for alkyl carbons and carbocations, when the carbon participates in pi-bonding (multiple bonding such as double or triple bonding), different names are applied.
2. Primary, Secondary, and Tertiary Carbocations
Carbocations can also be classified as primary, secondary, or tertiary according to the number of carbons directly attached to the positively charged carbon.
Quaternary carbocations don’t exist. The problem is that the extra p-orbital on carbon would bring the number of orbitals on carbon to 5, violating the octet rule.
Carbocations can also be classified as primary, secondary, or tertiary according to the number of attached carbons.
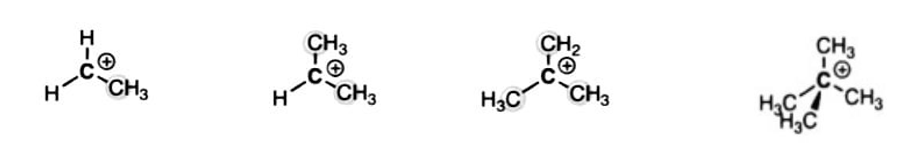
|
Primary (1°) carbocation |
Secondary (2°) carbocation |
Tertiary (3°) Quaternary (4°) carboncation carbocation |
|
-C attached to one carbon |
-C attached to two carbons |
-C attached to -does not exist three carbons -would require 5 orbitals at carbon, breaking octet rule |
3. Primary, Secondary, and Tertiary Alcohols
Primary, secondary, and tertiary alcohols are named according to the number of carbons directly attached to the C-OH carbon. This carbon is sometimes known as the carbinol carbon.
There is no such thing as a quaternary alcohol because that would require having 5 bonds to carbon.
Alcohols are classified as primary (1°), secondary (2°), or tertiary (3°) according to the number of carbons directly attached to the carbon bonded to the OH.
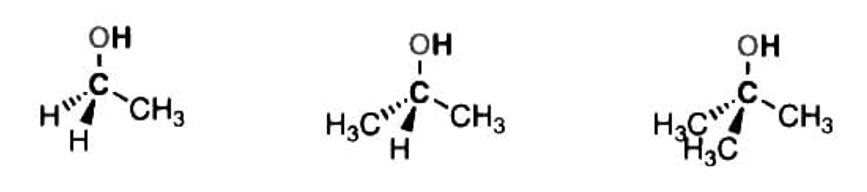
|
Primary (1°) alcohol |
Secondary (2°) Tertiary(3°) alcohol alcohol |
|
-C-OH carbon attached to one carbon |
-C-OH carbon -C-OH carbon attached to two carbons attached to three carbons |
4. Primary, Secondary, and Tertiary Alkyl Halides
Alkyl halides have an sp³ hybridized carbon directly attached to a halogen.
Like alcohols, they are named according to the number of carbons directly attached to the carbon containing the halogen.
Alkyl halides are classified as primary, secondary, or tertiary according to the number of carbons directly attached to the carbon bonded to the halogen.
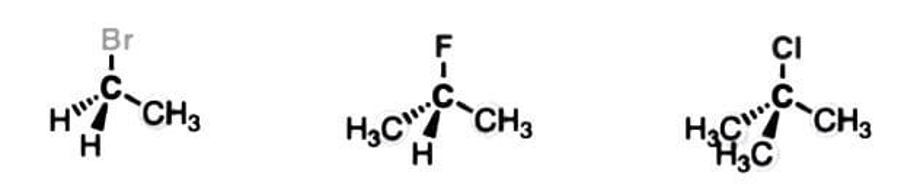
|
Primary Alkylhalide(1°) |
Secondary Alkylhalide(2°) |
Tertiary Alkylhalide(3°) |
|
-C-OH carbon attached to one carbon |
-C-OH carbon attached to two carbons |
-C-OH carbon attached to threerbons |
5. A Special Case: Methane and Methyl Groups
Up to this point we’ve left out the special case of methane, the only hydrocarbon which lacks any carbon-carbon bonds.
The Special case of methane

|
Methane |
Methyl Methyl Methyl carbocation alcohol halide |
|
-C attached to 0 carbon, 4 hydrogens |
-C attached to -C attached to -C attached to 0 carbon, 0 carbon, 0 carbon, 3 hydrogens 3 hydrogens 3 hydrogens |
6. Primary, Secondary, and Tertiary Amines and Quaternary Ammonium Salts
Next, we come to amines, Amines are named according to the number of carbons attached to the nitrogen. Primary, secondary, and tertiary amines are nitrogen bound to one, two and three carbons, respectively. It is possible for the nitrogen to be bound to a fourth carbon. This species is known as an alkylammonium salt. It is not technically an amine since it lacks a lone pair on nitrogen and cannot act as a base.
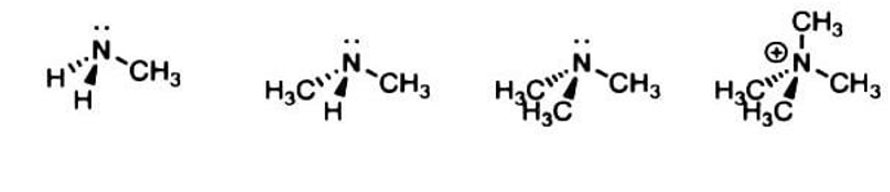
|
Primary (1°) amine |
Secondary (2°) Tertiary(3°) Quaternary (4°) amine amine amine |
|
-N attached to one carbon |
-N attached to -N attached to -N attached to two carbons three carbons four carbons |
Remember that the positive formal charge on nitrogen doesn’t imply that there is an empty p orbital there. Always assume a full octet on positively charged nitrogen and oxygen.