Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
0/1TOPIC – 03 : Stereoisomerism
Stereoisomerism
Isomers which have same structural formula but have different relative arrangement or atoms or groups in space are called Stereoisomers and the phenomenon is called Stereoisomerism.
This type of isomerism arises in compounds having the same chemical formula but different orientations of the atoms belonging to the molecule in three-dimensional space. The compounds that exhibit stereoisomerism are often referred to as stereoisomers. This phenomenon can be further categorized into two subtypes. Both these subtypes are briefly described in this subsection.
Geometric Isomerism :
- This type of isomerism is popularly known as cis-trans isomerism or E-Z isomerism.
- This isomers have different spatial arrangements of atoms in three-dimensional space.
- In the field of organic chemistry, cis isomers contain functional groups on the same side of the carbon chain whereas the functional groups are on opposite sides in trans isomers.
- This type of isomerism can arise in both organic and inorganic molecules.
- The prefix ‘cis’ and ‘trans’ have latin roots and can be translated as ‘the side of’ and ‘other side of’ respectively.
- Some coordination complexes have cis-trans isomers as well.
Examples of Cis-Trans Isomers :
Some examples of cis-trans isomers are provided in this subsection along with illustrations.
Organic Compounds :
The presence of a double or triple bond restricts the bond rotation within a molecule, which can lead to cis-trans isomerism. This type of isomerism can be observed in the organic compound but-2-ene.The structures of the cis and trans isomers of but-2-ene have been illustrated below.
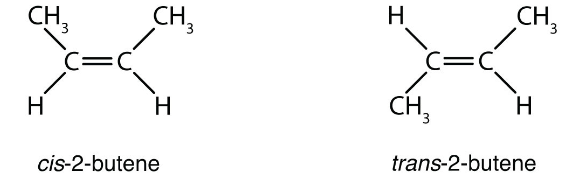
Another example of cis-trans isomerism of hex-3-ene is shown below.
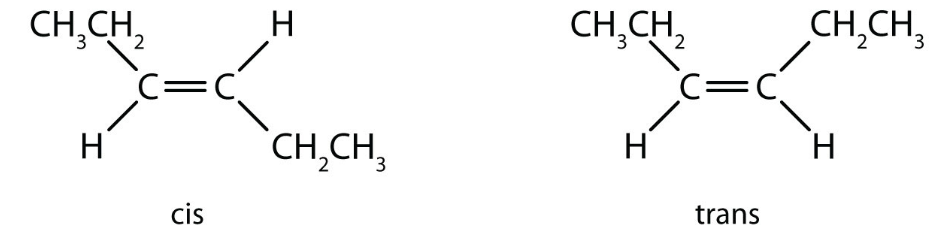
Inorganic Compounds :
Many diazenes and diphosphenes are known to have cis-trans isomers. Coordination complexes having square planar or octahedral geometries also display cis-trans isomerism based on the position of the ligands. The isomers of the coordination compound Pt(NH3)2Cl2 are illustrated below.
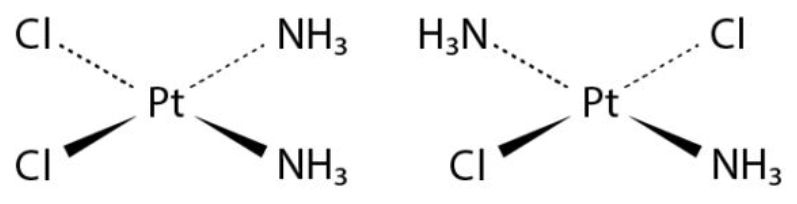
Optical Isomerism :
Optical isomers can be broadly classified into two types, namely enantiomers and diastereomers.
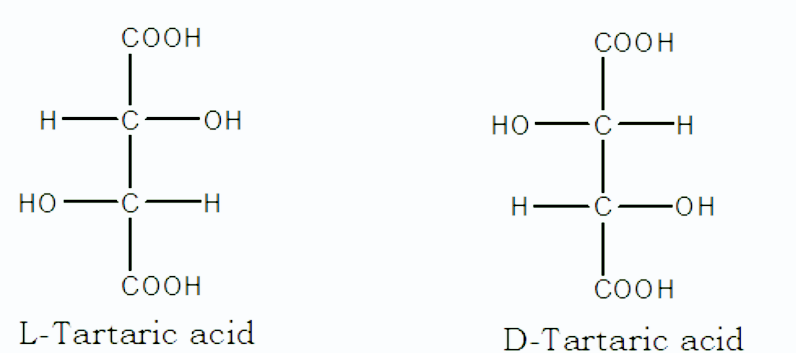
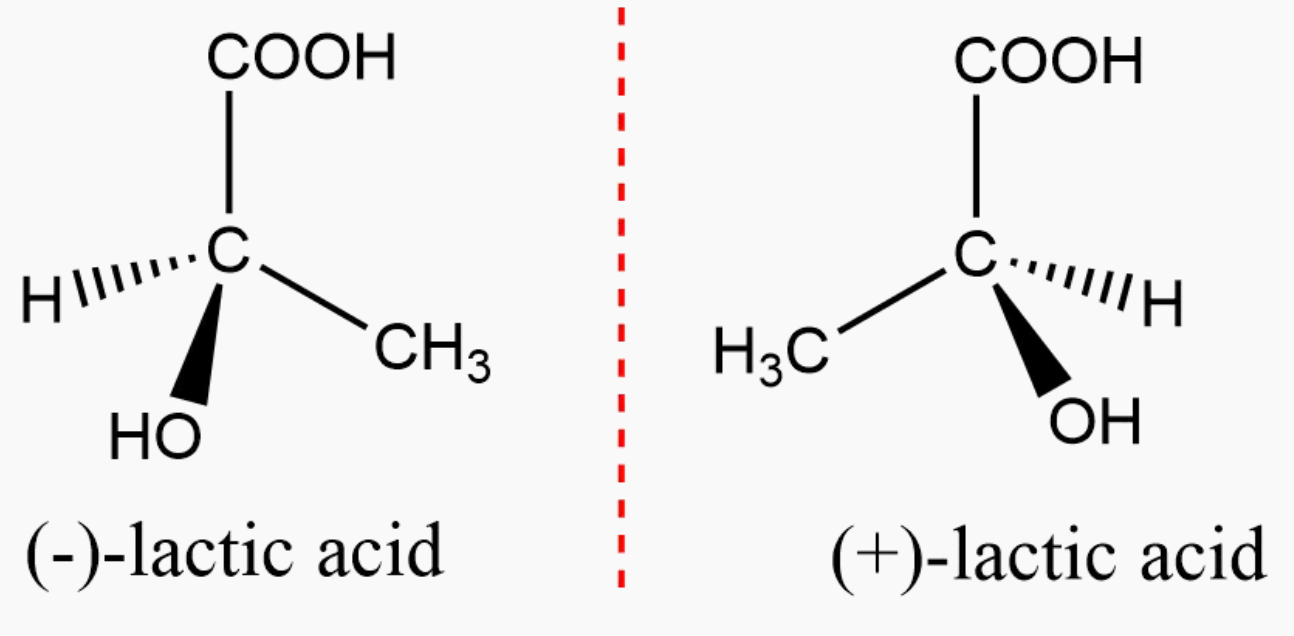
1. Enantiomers :
- When two isomers are mirror images of each other, that is, they are not superimposable to each other, the type of isomerism is called enantiomerism and those isomers are referred to as enantiomers.
- Enantiomers are stable and isolable compounds that differ in their spatial arrangements in three-dimensional space.
- They generally exist as discrete pairs.
- The properties of enantiomers are identical. However, their interaction with a plane of polarized light can vary.
- Their direction in which they rotate the plane-polarized light is different, that is, if one rotates in right direction, the other rotates towards left.
Example :
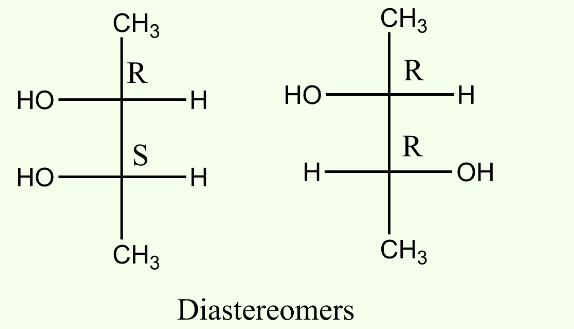
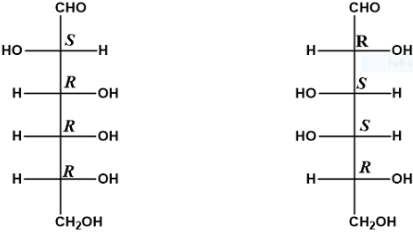
2. Diastereomers :
- When two isomers do not behave as mirror images of each other, they are called diastereomers.
- A molecule with n number of chiral carbon atoms can have up to 2n number of diastereomers.
- These isomers vary in physical properties and chemical reactivity.
- Diastereomers are also non-superimposable to each other.
- Diastereomers may or may not be optically active.
Example :