Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
0/1TOPIC – 01 : Why Organic Chemistry revolves around Carbon Compounds ?
Why Organic Chemistry revolves around Carbon Compounds ?
You are probably familiar with some forms of carbon. For example, the graphite of a pencil is one form of carbon. The carbon ( graphite ) of your pencil is very impotant, otherwise you didn’t see any writing. Also the paper you write on, is made up of carbon, not pure carbon but it has a lot of carbons in it.
Also, the diamonds you see, another form of carbon, it can take under intense time or after a long period of time under intense pressure.


But you may or may not realise, that carbon is actually essential for life. In fact, the life, that we know is carbon-based. When we are looking for signs of life, we are actually looking for carbon-based life. There might be other forms of elements that form the back-bone of life but carbon is the only one that we have been able to observe.
Now, this question might occure to you that why carbon forms the back-bone of life ? Why is carbon so valuable for life ?
Well ! It all comes down to the periodic table and sits in the atomic number and how it tends to bond with things. This is why chemistry is so important to us.
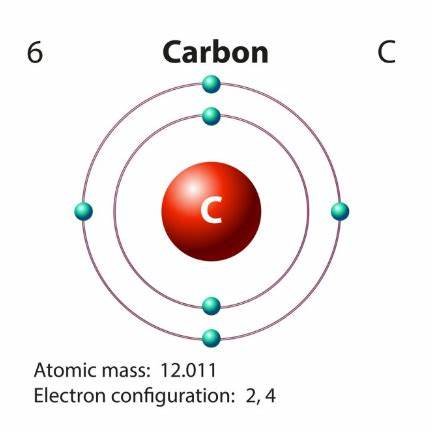
So, the carbon, we see over here, has an atomic number 6, by definition it has six protons in it. The most common isotope of carbon is C12 where it has six neutrons in the nucleus and also in the neutral carbon molecule, it has 6 electrons also. Out of the six electrons, two reside in the inner orbital and four reside in the outer orbital or in the valence shell. So, these four electrons in the valence shell are known as Valence Electrons.
As we are already aware about the octet rule, we know any molecule needs eight electrons in its outer orbital to be stable except hydrogen & helium.
So, carbon forms four covalent bonds with other atoms to gain eight electrons in its outer orbital to become stable like the nearest noble gas. For example, Hydrogen has one valence electron in its outer shell. So, carbon forms four covalent bonds with four hydrogen atoms each where both hydrogen and carbon share their electrons.
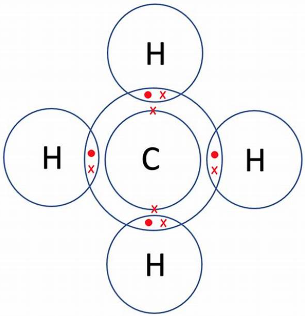
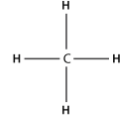
So, by definition, methane considered as an organic compound. And, the fact that methane has hydrogen and carbon in it, it is also considered to be a hydrocarbon. Other hydrocarbons we might come across in our daily life, are gasoline, petrolium etc.
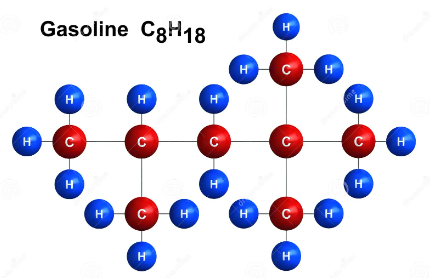
Figure : Petrolium’s constituent molecules
Actually these hydrocarbons are just long chains of carbons and hydrogens. Carbon is so useful that it can form so many bonds in different structures. These hydrocarbons may be in the structure of chains, rings or cycles and in diamonds or graphites, carbon can also form three dimensional lattice.
In these structures, carbon typically form bonds in tetrahedral shapes. When we think of a tetrahedron, it means, we are talking about a three dimensional shape with four sides and each side is a triangle.
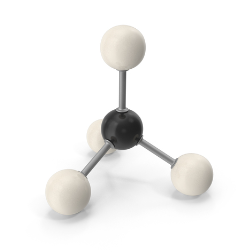
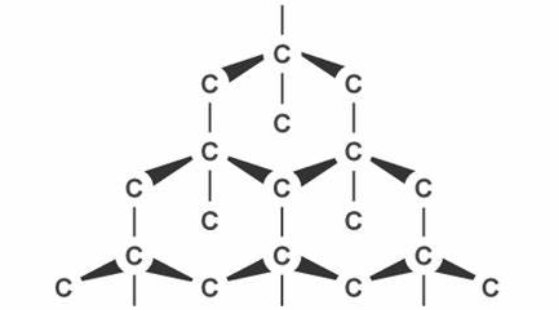
These three dimensional structures are actually the life forms as we know. Carbon forms almost 18% of our body mass. If we look at our DNA molecular structure, it’s all about carbon. Especially the most non-liquidy part of our body is carbon. To understand, if you simply look at your palm, you are actually looking at a lot of carbons.
Subtopic : Carbon is present in a little quantity in Earth’s crust when Silicon is the second most abundant element in Earth’s crust. Then why all the Organic Compounds are Carbon based and not Silicon based ?
Carbon alone can make C – C linkage. Carbon can easily form a single, double and triple bond. Besides, the bonding capabilities, carbon has a unique structure. Carbon has four valence electrons and therefore, makes four separate covalent bonds in compounds. Carbon has the ability to bond to itself repeatedly, making long chains of carbon atoms, as well as ringed structures. Carbon exists as pure form, whereas silicon exists as silicates in most cases, so, it is clear that carbon is readily available for reactions than that of silicon. Also, the polymerization property shown by carbon is not common in silicon, it does not polymerize. Silicon shows Si – O linkages in common than Si – Si linkages. That is the reason why most of the Organic Compounds are made of carbon and not silicon.
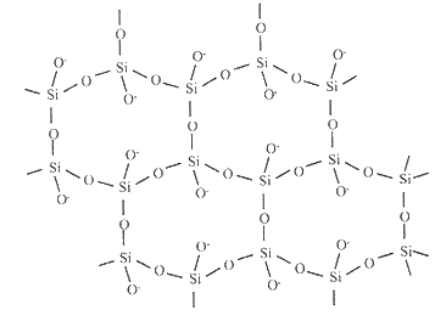

Figure : Si – O linkage Figure : Silica Gel