Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
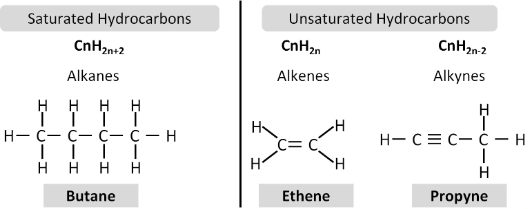
0/1TOPIC – 02 : Saturated and Unsaturated Hydrocarbon
Hydrocarbons are organic compounds consisting of carbon and hydrogen atoms only. In a saturated compound all the carbon–carbon bonds are single bonds. Unsaturated compounds contain double and/or triple carbon–carbon bonds. The simplest example of a saturated
hydrocarbon is methane, CH4, a member of the alkane family. Alkanes are aliphatic or straight-chain compounds.
The majority of naturally occurring hydrocarbons come from crude oil. This mixture is extracted from beneath the Earth’s surface, refined, and separated by fractional distillation into useful substances such as petroleum, butane, and kerosene.
The mixture of hydrocarbons that makes up crude oil is a combination of mainly alkanes, cycloalkanes and aromatic hydrocarbons.
Cycloalkanes are ring structures that contain single carbon–carbon bonds, whereas aromatic hydrocarbons or arenes are ring structures consisting of alternating single and double carbon–carbon bonds. Aliphatic or long chain hydrocarbons are divided into saturated and unsaturated compounds.