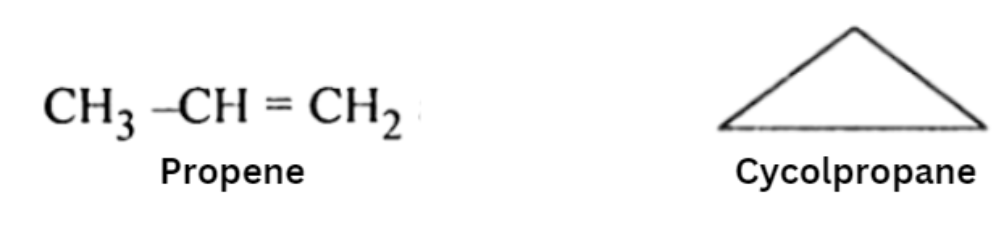Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
0/1TOPIC – 02 : Structural Isomerism
Structural isomers are those isomers in which the atoms are completely arranged in a different order with the same molecular formulas.
These are the molecules having the same kind of molecular formula with different connectivities depending upon the order they are put together. The structure of alkane (C4H10) is one of the simple examples representing a structural isomer with different isomers. With the increase in the number of carbon atoms in the alkane molecule, the structural isomers increase.
Structural Isomerism
The isomers differing in the atomic arrangement of the molecules without any kind of reference to the spatial arrangement are known as structural isomers. The phenomenon of these structural isomers is called structural isomerism.
Structural isomerism is also called constitutional isomerism, as per the IUPAC. It is a kind of isomerism where the molecules have the same molecular formula with different orders and bondings, as opposed to that of stereoisomerism.
Types of Structural Isomerism
There are three types of structural isomerism existing, namely chain isomerism, position isomerism and functional group isomerism.
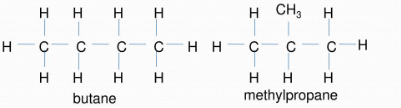
Chain Isomerism :
- Chain isomerism occurs when there is a difference in the atomic arrangement of the carbon to the carbon chain of a molecule.
- If two or more compounds have the same type of molecular formula with different main chains, then they are said to exhibit the property of chain isomerism. This phenomenon is also called skeletal isomerism.
Example :
For example, butane & methylpropane are chain isomers of each other. They have same chemical properties but different physical ones; the branched isomer has a lower boiling/melting point than the straight-chained one.
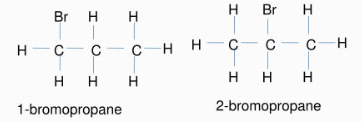
Position Isomerism :
- Positional isomerism arises when there is a difference in the positions occupied by the substituent atoms or a group of atoms or due to the unsaturation occurring in the chain.
- When the position of the functional groups with respect to the main chain atom changes, the phenomenon is called position isomerism.
Example :
For example, 1-bromopropane and 2-bromopropane are positional isomers of each other. They have similar chemical properties but different physical properties.
Functional Group Isomerism :
- Functional group isomerism occurs when there is a presence of an odd form of functional groups with the same chemical formula.
- When some compound has two different structures but the same chemical formula, then it is said to exhibit functional isomerism.
Example :
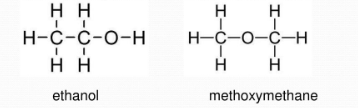
For example, ethanol and methoxymethane are functional group isomers of each other. They have the same molecular formula of C2H6O, however ethanol is an alcohol while methoxymethane is an ether.
Metamerism :
- This type of isomerism arises due to the presence of different alkyl chains on each side of the functional group.
- It is a rare type of isomerism and is generally limited to molecules that contain a divalent atom (such as sulphur or oxygen), surrounded by alkyl groups.
Example :
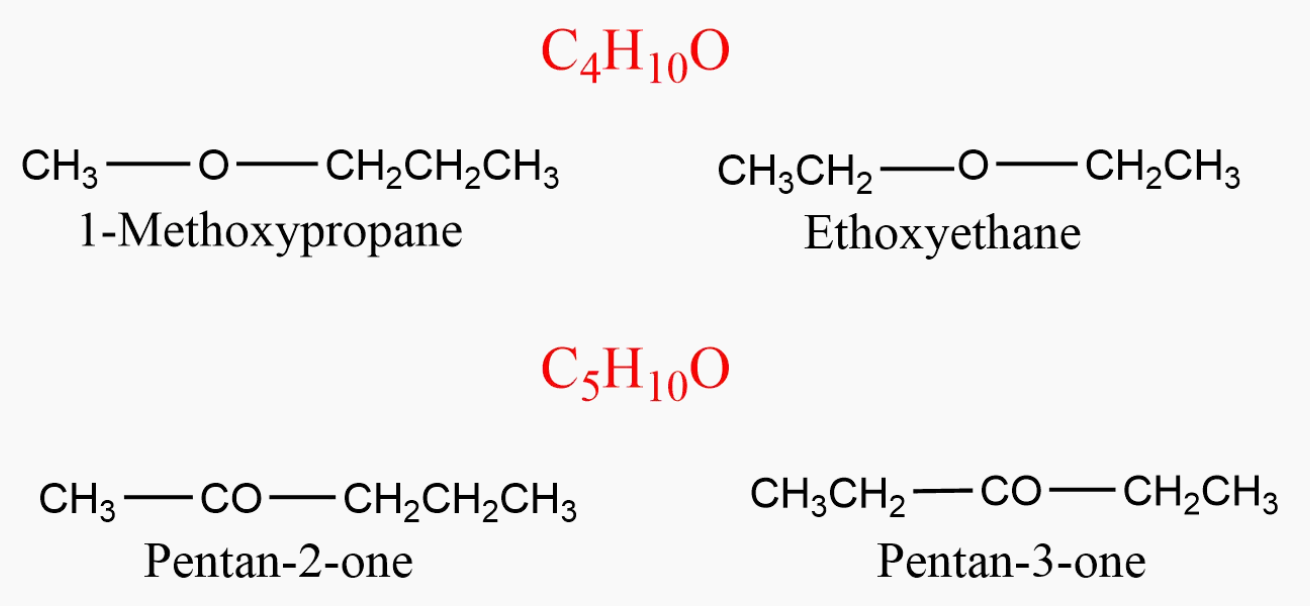
C4H10O can be represented as ethoxyethane (C2H5OC2H5 ) and 1-methoxypropane ( CH3OC3H7 ) & C5H10O can be represented as pentan-2-one (CH3COC3H7 ) and pentan-3-one (C2H5COC2H5 ).
Tautomerism :
- A tautomer of a compound refers to the isomer of the compound which only differs in the position of protons and electrons.
- Typically, the tautomers of a compound exist together in equilibrium and easily interchange.
- It occurs via an intramolecular proton transfer.
Example :
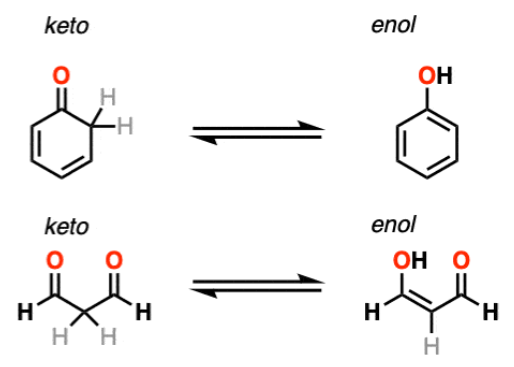
An important example of this phenomenon is keto-enol tautomerism.
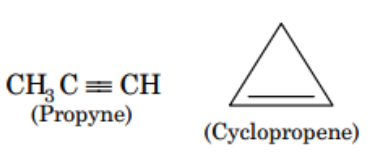
Ring-Chain Isomerism :
- In ring-chain isomerism, one of the isomers has an open-chain structure whereas the other has a ring structure.
- They generally contain a different number of pi bonds.
Example :
A great example of this type of isomerism can be observed in C3H6 ; propene and cyclopropane are the resulting isomers. Another example can also be observed in C3H4 where the resulting isomers are propyne and cyclopropene.