Curriculum
Fundamentals of Organic Chemistry
CONCEPT - 01 : General Introduction to Organic Chemistry
0/3ASSIGNMENTS : CONCEPT - 01
0/3CONCEPT - 02 : Structural Representation of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 02
0/3CONCEPT - 03 : Classification of Organic Compounds
0/2ASSIGNMENTS : CONCEPT - 03
0/2CONCEPT - 04 : Nomenclature of Organic Compounds
0/4ASSIGNMENTS : CONCEPT - 04
0/2CONCEPT - 05 : Homologous Series
0/4ASSIGNMENTS : CONCEPT - 05
0/2CONCEPT - 06 : Isomerism
0/3ASSIGNMENTS : CONCEPT - 06
0/2PRACTICE QUESTION SET
0/1TOPIC – 03 : Chemical formulae and Empirical formulae
Chemical formulae of organic compounds :
The structure of an organic compound may be represented in several different ways providing varying levels of information. We have already seen different ways to represent the chemical formulae for organic compounds i.e. Lewis ( electron-dot ) structure formulae, Complete structural formulae, Condensed structure formulae and Bond line or Skeletal formulae.
- Lewis sturctures are useful to visualize the valence electrons present in simple molecular compounds and polyatomic ions.
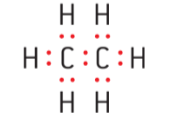

Figure 1 : Lewis structure of ethane ( C2H6 ) & methanoic acid ( HCOOH )
- Complete structural formulae are two-dimensional representations showing all the atoms and bonds, and their positions relative to one another in a compound.
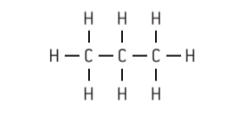
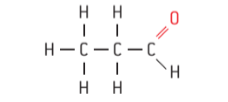
Figure 2 : Complete structural formulae of propane ( C3H8 ) & propanol ( CH3CH2CHO )
- In a condensed structural formula all the atoms and their relative positions are represented but the bonds are omitted.
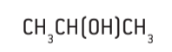

Figure 3 : Condensed structural formulae of propan-2-ol (C3H8O ) & propene ( C3H6 )
- A skeletal formula is the most basic representation of the structural formula where the carbon and hydrogen atoms are not shown but the end of each line and each vertex represents a carbon atom. The atoms present in functional groups are also included as shown in the below figures.
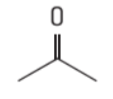
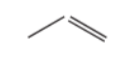
Figure 4 : Skeletal formulae of propanone ( C3H6O ) & propene ( C3H6 )
Empirical formulae :
Empirical formulae represent the simplest ratio of atoms present in a molecule. The molecular formula describes the actual number of atoms present in the molecule. Both these types of formula offer little or no information about the possible structure of larger, more complex molecules.
